This common bacteria is teaching scientists how to turn air into energy
A safe cousin of tuberculosis can turn hydrogen into its own fuel. Scientists have finally figured out how it works.
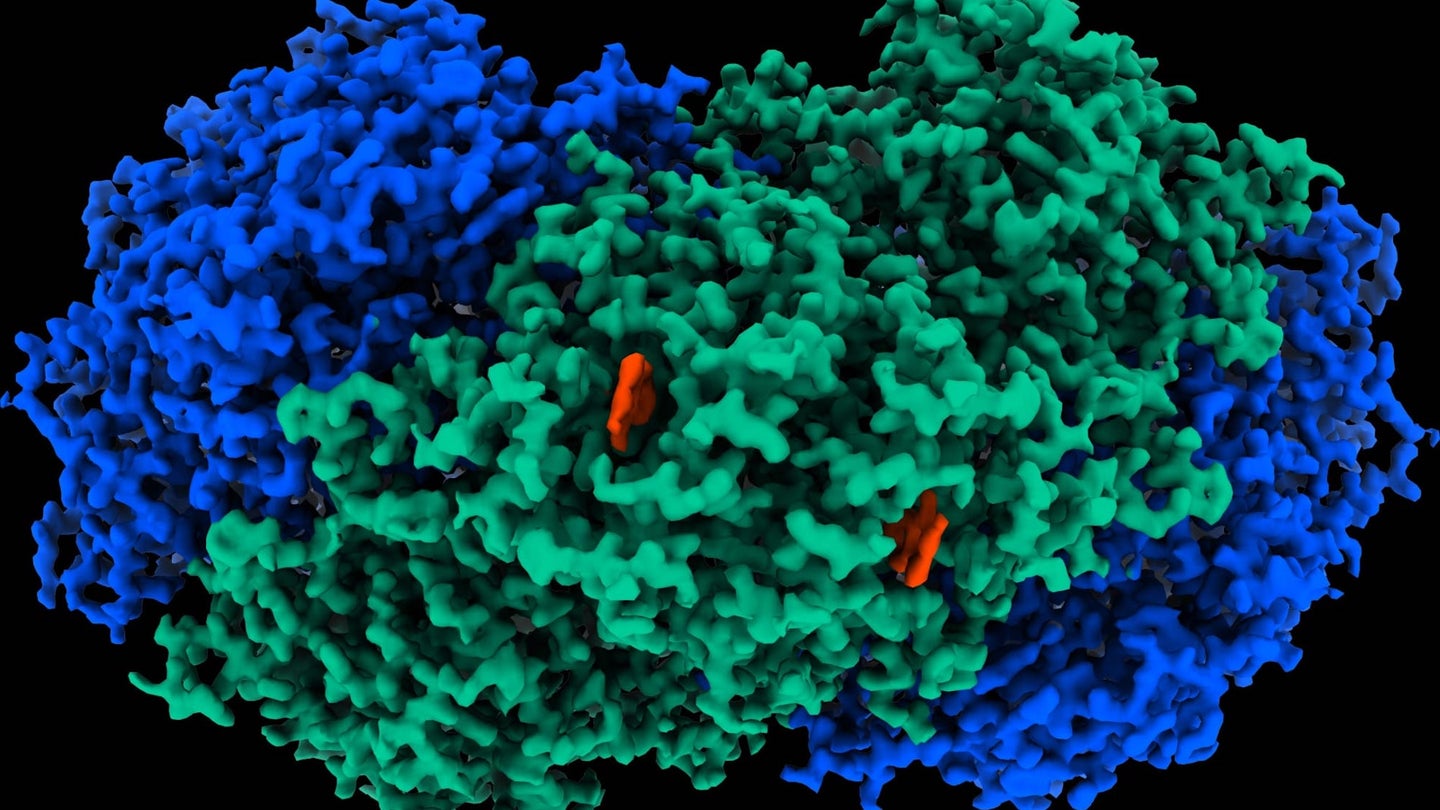
A bacterial relative of tuberculosis known as Mycobacterium smegmatis can pull off an incredibly impressive trick. When fuel is in short supply, it can absorb trace amounts of hydrogen in the atmosphere and water around it to convert into energy. Simply put, it turns air into electricity.
Unlike its infamous cousin, M. smegmatis is both nonpathogenic and commonly found in soil literally all over the world—from volcanic craters, to Antarctic climes, to the deepest ocean depths. This ubiquity and resilience is owed in part due to its ability to absorb miniscule levels of hydrogen for nutrition. Although researchers have been aware of the mechanism for some, they didn’t know how it worked. But as a new paper published in Nature reveals, the puzzle has finally been solved—and it could usher in a new era of revolutionary, clean energy.
Researchers at Australia’s Monash University Biomedicine Discovery Institute have discovered and isolated the M. smegmatis’ unique enzyme, dubbed “Huc,” enabling it to convert hydrogen into electricity. “Huc is extraordinarily efficient,” explains research co-lead and professor of microbiology, Chris Greening, in a statement last week. “Unlike all other known enzymes and chemical catalysts, it even consumes hydrogen below atmospheric levels—as little as 0.00005 percent of the air we breathe.”
[Related: Scientists think this tiny greenhouse could be a game changer for agrivoltaics.]
To isolate and identify the previously unknown enzyme, researchers utilized cryo-electron microscopy, which fired electrons at frozen Huc samples to map its atomic structure and electrical pathways. Another approach known as electrochemistry allowed researchers to demonstrate the purified enzyme could create electricity with only tiny concentrations of hydrogen. From there, researchers explained that by immobilizing Huc on an electrode, its electrons can subsequently transfer into an electrical circuit to generate current.
Although in its relative infancy, researchers hope the newly isolated Huc enzyme could one day be grown at scale, seeing as how M. smegmatis can be easily grown in large quantities within lab settings. What’s more, Huc isn’t alone in this ability. According to Monash researchers, between 60 and 80 percent of soil bacteria feature similar enzymes that collectively absorb 70 million metric tons of hydrogen per year. Further studies of these enzymes could provide insights into how to help stabilize atmospheric conditions in the face of climate change.
Before this, however, a natural Huc battery could be utilized akin to solar cells to eventually help power smartwatches, computers, or even one day cars. “Once we produce Huc in sufficient quantities, the sky is quite literally the limit for using it to produce clean energy,” said research co-lead Rhys Grinter, a research fellow at the Monash Biomedicine Discovery Institute and study co-lead, last week.
